Chapter: Pathophysiology & Classifcation of PoTS (2021) pt IV: MCAS & Immune-Mediated POTS
This chapter, in the book Postural Tachycardia Syndrome (2021), written by Matthew G. Lloyd & Satish R. Raj, talks about subtypes of Postural Orthostatic Tachycardia Syndrome (POTS). I’ve previously covered Neuropathic POTS, Hypovolemic POTS, and Hyperadrenergic POTS.
The Paper
Immune-Mediated
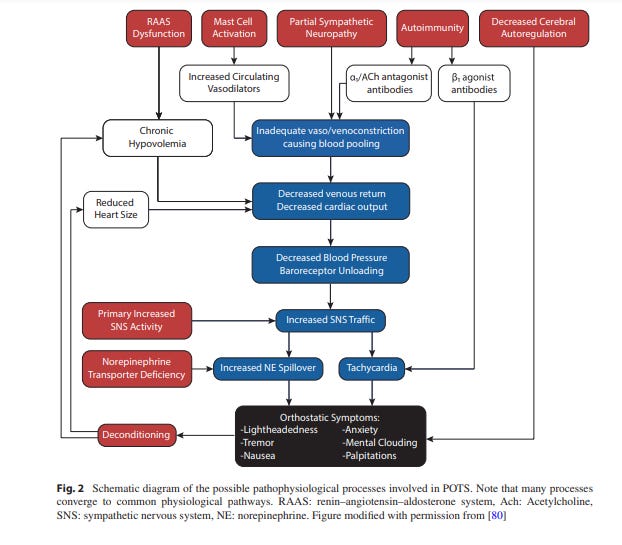
The immune system is super likely to be involved in POTS.
For one, many cases of POTS happen after an acute viral illness or vaccination, 1 2 which is similar to autoimmune neurological disorders (like Guillain-Barre syndrome).
Second, there’s huge overlap between symptoms of POTS & autoimmune disorders or viral syndromes (e.g. myalgias, fatigue, gastrointestinal issues, and nausea).
This is especially true of POTS & Sjögren’s Syndrome, an autoimmune disorder that “primarily affects exocrine glands, resulting in dry eyes and dry mouth.”
A recent case series of 13 Sjögren’s patients at the Arizona Mayo Clinic’s autonomic clinic found that 62% also had POTS.3
Antibodies
Various antibodies have been found in some POTS patients, which contributes to their POTS:4 5
Alpha-1 adrenergic receptor antibodies, which might “reduce sympathetically-mediated vasoconstriction” and therefore cause the body to need to increase sympathetic activity to maintain blood pressure (BP).
β2 adrenergic receptor antibodies may also “have an agonist [stimulating] effect to the vasodilatory function of the receptor” and thus also require an increase of sympathetic activity to maintain BP.
β1 adrenergic receptor antibodies, which provides a “direct mechanism for orthostatic tachycardia”
Low titer of ganglionic acetylcholine receptor antibodies have been found in 5-29% of POTSies.6 7 8 (but the clinical relevance is debated, as these can also be found in healthy controls, too)
+ Sundry antibodies against: cardiac lipid raft-associated proteins9, muscarinic receptors 1 and 210, and the angiotensin II type 1 receptor11
Mast Cell Activation
Part of the “wide symptomatology” of POTS “includes “allergic-like” reactions to food, odors, and medications.” This has lead to the investigation of mast cells as possible contributors to POTS.
“Mast cells are white blood cells that reside in close proximity to blood vessels and peripheral nerves.12 They are involved in a variety of immune responses, such as neuro-immune responses, autoimmunity, and allergic reactions.13”
Mast cell activation disorders (MCADs) is the umbrella term for disorders involving mast cell tomfoolery. It includes
MCAS in particular has been connected to PoTS.
Our authors define MCAS thusly:
“MCAS is characterized by the following criteria:
(1) typical symptoms (flushing, lightheadedness, chest pain, rapid heart rate, muscle and bone pain, diarrhea, dizziness/vertigo, etc.);
(2) a substantial increase (>20%) in a mast-cell-derived mediator (e.g. serum total tryptase, urinary methylhistamine, urinary prostaglandin D2, etc.), during or shortly after a symptomatic event, and
(3) a response of clinical symptoms to H1- and H2-histamine receptor blockers, high dose aspirin, cromolyn-based agents, or other mast cell targeting agents.14”
Elsewhere in the literature:
Apparently, there’s almost nothing in the literature right now about POTS + MCAS.
In fact, there’s apparently only 1 study directly looking at the prevalence of MCAS in POTS. In it, 8 out of 24 POTS patients had MCAS (“defined as a history of facial or upper trunk flushing, and urine methylhistamine>230 µg/g”). These patients were hyperadrenergic and had “exaggerated sympathetic pressor response“ to the Valsalva maneuver.
“The purported mechanism linking mast cell activation with PoTS is via increased histamine release from mast cells, which causes
systemic vasodilation,
an excessive baroreflex-mediated increase in sympathetic activity to maintain BP,
and associated tachycardia.15”
At least one study did examine rates of dysautonomia in patients with hypertryptasemia and found that 46% of these patients had autonomic dysfunction.16 17
According to the authors, there’s not been any studies “officially” examining the prevalence of POTS in MCAS patients. However,
One study reported that 71% of mastocytosis/MCAS had daily or occasional light-headedness
And a lot of MCAS symptoms significantly overlap with POTS (e.g. “brain fog, anxiety, diarrhea, headache, bloating, joint pain, etc.”)
Despite having so few papers discussing the MCAS & POTS connection directly, the “the bi-directional interaction between mast cells and neurons provide a strong basis of plausibility for a pathophysiological link” (aka mast cells and neurons are totes able to mess each other up).
Many allergic reaction symptoms are largely mediated by neuronal activity (eg “itchy eyes, bronchospasm, and mucous production”)18
“Neurons can activate mast cells via the release of substance P and calcitonin gene-related peptide” 19 20
Mast cells can affect neurons via the stuff they release, too (“mediators”), such as: “tryptase, cysteinyl leukotrienes, prostaglandins, tumor necrosis factor α, neurotrophin, and nerve growth factor.” 21
Next up, the final section: Impaired Cerebral Autoregulation & Deconditioning!
Lloyd MG, Raj SR. Pathophysiology and classification of pots. In: Gall N, Kavi L, Lobo MD, eds. Postural Tachycardia Syndrome. Springer International Publishing; 2021:29-40. doi:10.1007/978-3-030-54165-1_5
Comments are open for discussion, as well as if you have a correction or clarification of anything in this post!
Citations:
Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993 [cited 2019 Apr 27];43:132–7. https://www.ncbi.nlm.nih. gov/pubmed/8423877.
Vernino S, Stiles LE. Autoimmunity in postural orthostatic tachycardia syndrome: current understanding. Auton Neurosci Basic Clin. 2018;215:78– 82. https://doi.org/10.1016/j.autneu.2018.04.005.
Goodman BP, Crepeau A, Dhawan PS, Khoury JA, Harris LA. Spectrum of autonomic nervous system impairment in Sjögren syndrome. Neurologist. 2017;22:127–30.
Fedorowski A, Li H, Yu X, Koelsch KA, Harris VM, Liles C, et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace. 2017;19:1211–9.
Li H, Yu X, Liles C, Khan M, Vanderlinde-Wood M, Galloway A, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3:1–10.
Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, et al. Postural orthostatic tachycardia syndrome: the mayo clinic experience. Mayo Clin Proc. 2007;82:308–13.
Vernino S, Hopkins S, Okamoto L, Black B, Paranjape SY, Raj SR. Prevalence of ganglionic AChR antibodies in postural tachycardia syndrome (POTS). Clin Auton Res. 2016;26:328.
Watari M, Nakane S, Mukaino A, Nakajima M, Mori Y, Maeda Y, et al. Autoimmune postural orthostatic tachycardia syndrome. Ann Clin Transl Neurol. 2018;5:486–92.
Wang XL, Ling TY, Charlesworth MC, Figueroa JJ, Low P, Shen WK, et al. Autoimmunoreactive IgGs against cardiac lipid raft-associated proteins in patients with postural orthostatic tachycardia syndrome. Transl Res. 2013;162:34–44. https://doi. org/10.1016/j.trsl.2013.03.002
Vernino S, Stiles LE. Autoimmunity in postural orthostatic tachycardia syndrome: current understanding. Auton Neurosci Basic Clin. 2018;215:78– 82. https://doi.org/10.1016/j.autneu.2018.04.005.
Yu X, Li H, Murphy TA, Nuss Z, Liles J, Liles C, et al. Angiotensin II type 1 receptor autoantibodies in postural tachycardia syndrome. J Am Heart Assoc. 2018;7:1–7.
Frieri M. Mast cell activation syndrome. J Allergy Clin Immunol. 2018;54:353–65.
Valent P, Akin C, Arock M, Brockow K, Butterfeld JH, Carter MC, et al. Defnitions, criteria and global classifcation of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–25.
Ibid (same as 13)
Shibao C, Arzubiaga C, Roberts LJ, Raj S, Black B, Harris P, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension. 2005;45:385–90.
Lyons JJ, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, et al. Elevated basal serum tryptase identifes a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564–9.
Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: A refned and abbreviated composite autonomic symptom score. Mayo Clin Proc. 2012;87:1196–201. https://doi.org/10.1016/j. mayocp.2012.10.013.
Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. 2014;133:1521–34. https://doi. org/10.1016/j.jaci.2013.11.027.
Keller JT, Marfurt CF. Peptidergic and serotoninergic innervation of the rat dura mater. J Comp Neurol. 1991;309:515–34.
Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci. 1987;84:2975–9.
Mittal A, Sagi V, Gupta M, Gupta K. Mast cell neural interactions in health and disease. Front Cell Neurosci. 2019;13:1–6.